J. Neurosci. 2014;34(4):1280-92. 10.1523/JNEUROSCI.3046-13.2014
Firing of hippocampal neurogliaform cells induces suppression of synaptic inhibition.
External link to editorial:
Full text PDF download:
Abstract:
Little is known about how neuron firing recorded in vivo retrogradely influences synaptic strength. We injected the firing of a rat hippocampal neurogliaform cell (NGFC), a widely expressed GABAergic neuron type, detected in vivo during theta rhythm, into NGFCs of rat or neuronal nitric oxide synthase (nNOS)-Cre-tdTomato mouse recorded in vitro. We found that the "in vivo firing pattern" produced a transient firing-induced suppression of synaptic inhibition (FSI) evoked by a presynaptic NGFC. Imaging experiments demonstrate that FSI was associated with action potential backpropagation (bAP) and a supralinear increase in dendritic Ca(2+). The application of the L-type Ca(2+) channel antagonist nimodipine blocked FSI. Further pharmacological experiments, such as the application of a nitric oxide-sensitive guanylyl cyclase (NO-sGC) receptor antagonist, a NOS inhibitor, and NO donors, suggested that NO released from postsynaptic cells mediated FSI and likely activated presynaptic receptors to inhibit GABA release. The in vivo firing pattern modulated the size of unitary EPSPs impinging on NGFCs through FSI and not via a direct effect on excitatory synaptic transmission. Our data demonstrate: (1) retrograde signaling initiated by in vivo firing pattern, (2) interneuron bAPs detected with fast temporal resolution, and (3) a novel role for NO expressed by specific interneuron types.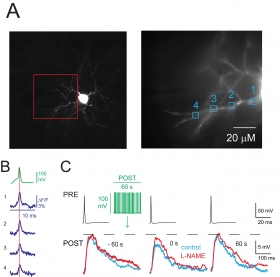 A) Left, image of an NGFC showing soma and characteristic stellate dendrites. This neuron was loaded with JPW-1114 and voltage imaging was performed from dendritic sites included in the red box; ΔF/F signals at the sites indicated (1-4, right picture) were analyzed. B) Depolarizing current pulse induced a somatic action potential (AP) recorded in current clamp (green) and the corresponding ΔF/F signals from the sites 1-4 (blu) are illustrated. Data not shown here suggest that AP back-propagation triggers increase of calcium concentration and release of nitric oxide from dendrites. C) Presynaptic APs (PRE, black traces) elicited depolarizing uIPSPs (control, left traces superimposed) recorded with an electrode filled with high Cl- solution showing FSI immediately after stimulation protocol (green traces) applied to a postsynaptic NGFC (control, blue middle trace). The uIPSP recovered 60 s after the stimulation protocol (blue trace, right). Application of the nNOS inhibitor L-NAME (200 μM) blocked FSI (red traces).
A) Left, image of an NGFC showing soma and characteristic stellate dendrites. This neuron was loaded with JPW-1114 and voltage imaging was performed from dendritic sites included in the red box; ΔF/F signals at the sites indicated (1-4, right picture) were analyzed. B) Depolarizing current pulse induced a somatic action potential (AP) recorded in current clamp (green) and the corresponding ΔF/F signals from the sites 1-4 (blu) are illustrated. Data not shown here suggest that AP back-propagation triggers increase of calcium concentration and release of nitric oxide from dendrites. C) Presynaptic APs (PRE, black traces) elicited depolarizing uIPSPs (control, left traces superimposed) recorded with an electrode filled with high Cl- solution showing FSI immediately after stimulation protocol (green traces) applied to a postsynaptic NGFC (control, blue middle trace). The uIPSP recovered 60 s after the stimulation protocol (blue trace, right). Application of the nNOS inhibitor L-NAME (200 μM) blocked FSI (red traces).