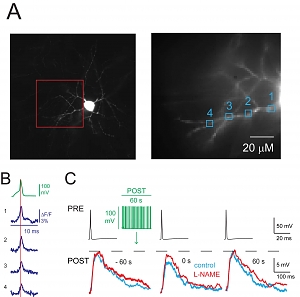
 A) Left, image of an NGFC showing soma and characteristic stellate dendrites. This neuron was loaded with JPW-1114 and voltage imaging was performed from dendritic sites included in the red box; ΔF/F signals at the sites indicated (1-4, right picture) were analyzed. B) Depolarizing current pulse induced a somatic action potential (AP) recorded in current clamp (green) and the corresponding ΔF/F signals from the sites 1-4 (blu) are illustrated. Data not shown here suggest that AP back-propagation triggers increase of calcium concentration and release of nitric oxide from dendrites. C) Presynaptic APs (PRE, black traces) elicited depolarizing uIPSPs (control, left traces superimposed) recorded with an electrode filled with high Cl- solution showing FSI immediately after stimulation protocol (green traces) applied to a postsynaptic NGFC (control, blue middle trace). The uIPSP recovered 60 s after the stimulation protocol (blue trace, right). Application of the nNOS inhibitor L-NAME (200 μM) blocked FSI (red traces).
A) Left, image of an NGFC showing soma and characteristic stellate dendrites. This neuron was loaded with JPW-1114 and voltage imaging was performed from dendritic sites included in the red box; ΔF/F signals at the sites indicated (1-4, right picture) were analyzed. B) Depolarizing current pulse induced a somatic action potential (AP) recorded in current clamp (green) and the corresponding ΔF/F signals from the sites 1-4 (blu) are illustrated. Data not shown here suggest that AP back-propagation triggers increase of calcium concentration and release of nitric oxide from dendrites. C) Presynaptic APs (PRE, black traces) elicited depolarizing uIPSPs (control, left traces superimposed) recorded with an electrode filled with high Cl- solution showing FSI immediately after stimulation protocol (green traces) applied to a postsynaptic NGFC (control, blue middle trace). The uIPSP recovered 60 s after the stimulation protocol (blue trace, right). Application of the nNOS inhibitor L-NAME (200 μM) blocked FSI (red traces). The hippocampus contains more than 20 types of inhibitory interneurons that express different proteins and impinge on different regions of pyramidal cells to regulate spatiotemporal integration of EPSPs and define temporal windows for spiking. Neurogliaform cells (NGFCs) form synapses on the distal tufts of pyramidal cell apical dendrites alongside excitatory inputs from the entorhinal cortex. NGFCs express neuronal nitric oxide synthase (nNOS), are often synaptically coupled, and fire during theta oscillations in vivo. Li et al. published a “featured article” in the Journal of Neuroscience (34(4):1280-1292, 2014) reporting a novel physiological action mediated by this interneuron type. They found that when theta-associated activity patterns were evoked in NGFCs in hippocampal slices of rat or mouse, the cells showed a transient reduction in unitary IPSP amplitude. This “firing-induced suppression of inhibition” (FSI) required back-propagation of action potentials, calcium influx through L-type calcium channels, nNOS activity, and activation of NO-sensitive guanylyl cyclase (NO-sGC) receptors, which are present on presynaptic terminals. FSI also indirectly increased the amplitude of EPSPs. Thus FSI may enhance spatial and temporal summation of excitatory inputs to NGFCs, regulating their inhibition of pyramidal cells. More in general, this work demonstrates: 1) retrograde signaling initiated by “in vivo firing pattern”, 2) interneuron back-propagation detected with fast time resolution voltage imaging, and 3) physiological role for nNOS expressed by specific interneuron types.